The 2nd law of thermodynamics can be described by two classical statements. One of these statements is called the Kelvin-Planck statement. This statements is related to heat engines. In addition to the Kelvin-Planck statement, the the Clausius statement also describes the 2nd law. This statement is related to refrigerators and heat pumps.
Kelvin-Planck Statement
First, the Kelvin-Planck statement is expressed as the following.
“It is impossible for any device that operates on a cycle to receive heat from a single reservoir and produce a net amount of work.”
As a result, a heat engine must exchange heat from a high temperature source to a low temperature sink.
Clausius Statement
Next, the Clausius Statement is expressed as the following.
“It is impossible to construct a device that operates in a cycle and produces no effect other than the transfer of heat from a lower-temperature body to a higher temperature body.”
In other words, this statement is acknowledging that heat will not transfer from a cold medium to a warm one under its own volition. However, it does not say that such a device is not impossible to construct. Instead it is stating that in order for this occur work must implied. For a refrigerator, or heat pump, work is applied using a compressor that is driven from an external power source.
Comparing the Two Statements
Both the Kelvin-Planck Statement and Clausius Statement are negative statements. A negative statement is a statement that cannot be proved. However, to this date there has not been an experiment that disproves the 2nd law of thermodynamics. As a result, this is taken as sufficient proof of validity. Due to this fact if a device violates the Clausius Statement than it will also violate the the Kelvin-Planck Statement, or vise versa.
For example let consider a heat-engine-refrigerator combination that is shown in the following images.
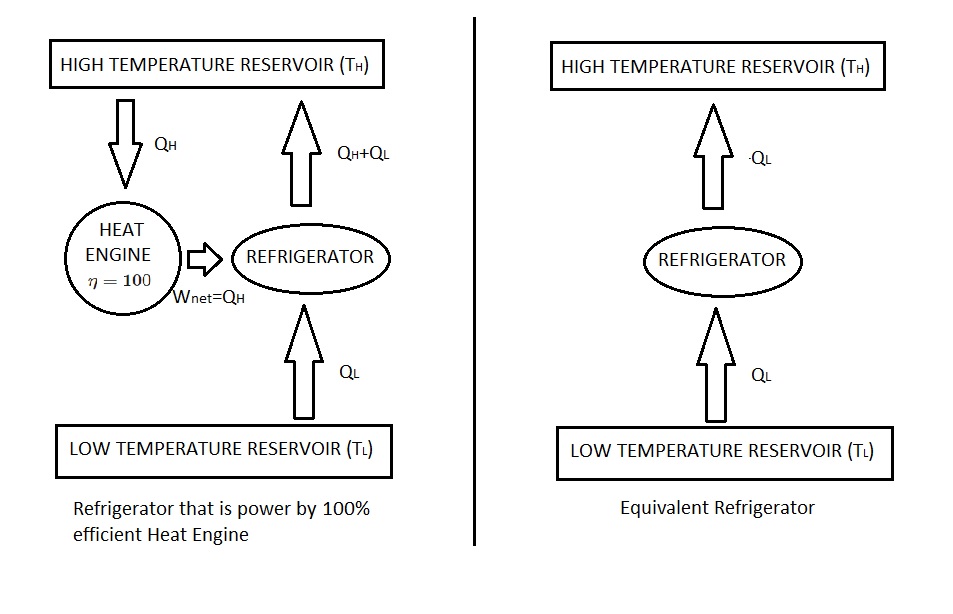
First, let’s take a look at the Kelvin-Planck statement. According to this statement a heat engine cannot have 100% efficiency. Instead it must exchange a certain amount of heat from a high temperature source to a low temperature sink. However, the image is showing that the heat engine is converting all of $Q_H$ to work which can only occur if it has 100% efficiency. Hence, it is in violation of the Kelvin-Planck statement.
Next, let’s take a look a the Clausius statement. In order to respect this statement work energy from an outside source must be applied. According to the image, all of $Q_H$ is converted to work. This is than used to run the refrigerator to remove a certain amount of heat $Q_L$ from the low temperature reservoir. By doing this $Q_L+Q_H$ will be rejected to the high temperature reservoir. In turn, the high temperature reservoir receives a net amount of heat $Q_L$. This means that work is not required to run this refrigerator, violating Clausius statement.
As shown, if the Clausius statement is violated than the Kelvin-Planck statement is also violated. This means that both statements are equivalent expressions for the second law of thermodynamics.