Any substance that has a fixed chemical composition is called a pure substance. This includes, but not limited to water, nitrogen, oxygen, and carbon dioxide. In addition, a pure substance does not have to be single element or compound. Instead a mixture that is homogeneous, such as air, can also be considered a pure substance. On the other hand, if you were to mix water and oil you would not obtain a pure substance. This is because oil is not soluble in water. Finally, a mixture of two are more phases of a pure substance will remain a pure substance as long as all phases have the same chemical composition. An example of this is ice mixed with water.
Phases of a Pure Substance
All substance exist in different phases that are dependent on temperature and pressure. The three principle phases are solid, liquid, and gas.
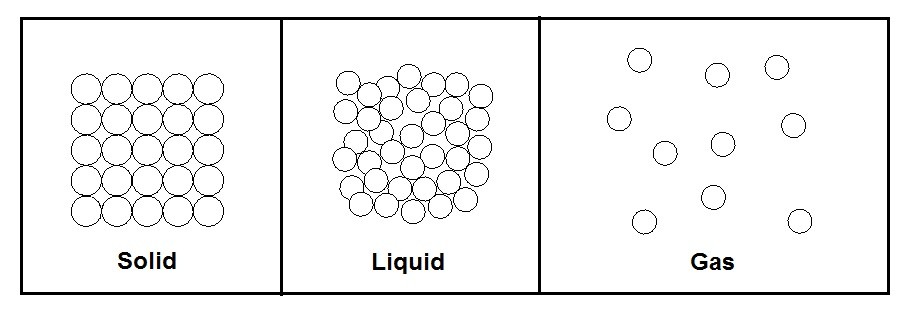
Solid
A solid has the strongest intermolecular bonds. In addition, these bonds are arranged in a three-dimensional pattern that is repeated throughout the solid. Finally, since the molecules are so close together, the attractive force between the molecules is large. As a result, the molecules will be kept in a fixed position.
There is also repulsive force between the molecules. This force will increase as the distance between them approaches zero. In turn, this prevents the molecules from piling up on one another.
Finally, even though the molecules within a solid cannot move around each other they still continually oscillate about their equilibrium positions. The rate of oscillation is dependent on the temperature. As a result, if there is a high enough temperature, the momentum from the molecule isolation will overcome the intermolecular forces. When this occurs the melting process of the solid has started. There are also cases where a solid molecule might have so much energy that it skips the melting process and goes directly to the gas phase. This is known as sublimation.
Liquid
The next phase that a substance can be is a liquid. During the liquid phase the spacing between the molecules is not much different in comparison to a solid. However, unlike a solid the molecules are no longer fixed as a lattice. Instead the molecules can now rotate and translate freely. As a result, they can now flow over each other.
Gas
The final phase that a substance can be is a gas. A gas has the weakest intermolecular bonds. As a result, molecules will be spread far apart from one another. They also will move around at random colliding into each other and the wall of the their container.
During the gas phase molecules are at a significant higher energy level. Due to this fact, a large amount of energy will need to be released from the gas for it to enter the liquid or solid phase.
In addition, to the three main phases of a substance, it is possible for a substance to have several phases within one of these three principle phases. An example of this is carbon. When carbon solidifies, depending on the conditions that caused the carbon to solidify, it could become a graphite or it could become a diamond. These phases within a principle phase are dictated by a distinct molecular arrangement that the molecules take. However, in order for these phases to result in a pure substance, the molecular structure must be homogeneous.
Phase Change Processes
There are many situations where a pure substance is in equilibrium as two phases. For example within a boiler water will exist as both vapor and a liquid.
Compressed Liquid and Saturated Liquid
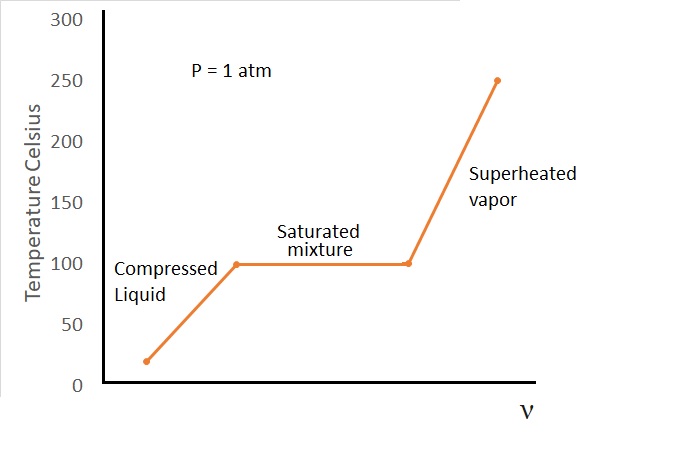
A compressed liquid is a liquid that is not about to vaporize. It is also sometimes referred to as a subcooled liquid. An example of a compressed liquid would be water that is at $20^oC$ and $1~atm$ of pressure.
Now let’s say the water is placed inside a piston while it is at $20^oC$. If the temperature of the water was than increased, the volume of the water will slightly increase, increasing its specific volume. As a result, the piston will have to move slightly due to the increase in volume of the water. This will allow the pressure inside the piston to remain at $1~atm$.
Finally, if the temperature of the water were to increase to $100^oC$ at a pressure of $1~atm$, it will reach the point where the water is still a liquid, but any added energy will cause it to start to vaporize. In turn, this is called a phase change process. In addition, at this point the water is no longer a compressed liquid. Instead the water is saturated liquid.
Saturated Vapor and Superheated Vapor
At the point where a liquid starts to boil, the temperature of the pure substance will stop rising until the liquid has been completely vaporized. In other words, as long as the pressure is held constant, the temperature will remain constant during the phase change process. Once all of the liquid has been evaporated the vapor will become a saturated vapor. A saturated vapor is a vapor that is about to condense back into a liquid. If the there is still liquid within the container than we would have what is called a saturated liquid-vapor mixture.
If energy is further transferred to the vapor after all of the liquid has evaporated the temperature of the vapor will continue to increase. At this point we will have a superheated vapor. In order for the vapor to be super heated it must not be at the verge of condensing. In other words, if we were to remove some heat from the vapor, it would not immediately enter a phase change condition where it starts to condense back into a liquid.
The figure below shows a t-ν diagram of water at $1~atm$ of pressure.
Saturation Temperature and Saturation Pressure
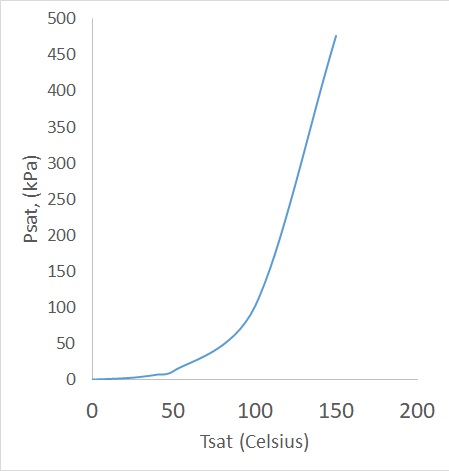
There is a point where all pure substances will start to boil. For water most people would say the boiling point is at $100^oC$. However, this is only partially correct. Water boils at $100^oC$ when the atmospheric press is $1~atm$. If you were to change the pressure the boiling temperature will also change. For example if the pressure were to change to $500~kPa$, which is about $5~atm$, the boiling temperature for water will change to $151.8^oC$.
Now if the we know the pressure is going to be constant, the temperature in which a phase change will occur is called the saturation temperature, $T_{sat}$. On the other hand, if the temperature were to stay constant and the pressure were to vary than the pressure at which a phase change occurs is called the saturation pressure $P_{sat}$.
The figure below shows the liquid-vapor saturation curve of water.
In order to melt a solid or vaporize a liquid a certain amount of energy is required. This energy is called the latent energy. To be more specific the energy required to melt a solid is called the latent heat of fusion. In addition, the energy required to vaporize a liquid is called the latent heat of vaporization.
$T_{sat}$ and $P_{sat}$ Dependence
Since a substance will boil at a temperature that corresponds to a pressure, we can control the temperature that substance would boil by controlling the pressure. There are a numerous amount of applications that take advantage of this fact.
For example lets consider a can of refrigerant. The refrigerant is initially at room temperature. However, the pressure inside the can is significantly higher than the pressure outside of it. Now if we were to open the can of refrigerant the liquid inside will start to vaporize. In turn, this will cause the can to cool. The reason for this is because at $1~atm$ the saturation temperature of the refrigerant, if it is refrigerant 134a, is $-26^oC$. It will remain at this temperature until all of it has vaporized causing the temperature of the can to drop.
This same phenomenon can be used to vacuum cool vegetables. In order to do this the pressure inside the a sealed chamber is reduced. In turn, this will change the saturation pressure of water to a lower temperature. As a result, the water will start to evaporate which will pull latent heat from the vegetables cooling them. Finally, this method can be taken to the point of freezing, which than it becomes vacuum freezing.