Energy is present in almost all of aspects of life. It is what provides us with sustenance. In addition, it can improve our quality of life. Due to this fact as an engineer it is important to have an understanding of what energy is. As well as what different sources of energy are, and how those sources of energy can be converted to different forms of energy.
Forms of Energy
So what are the forms of energy? There are numerous forms. To name a few there is mechanical, chemical, electrical, and thermal. There are two distinct types of energy; work and heat. Both work and heat can be transferred to and from a closed system. In addition, they can be transferred by mass flow through a control volume. If there is temperature difference the energy that is transferred takes the form of heat. Otherwise the transferred energy is work.
Conservation of Energy
As stated by the conservation of energy principle, energy cannot be created or destroyed. Instead energy can only change forms. This is the basis of the first law of thermodynamics.
To truly understand conservation of energy let’s consider this example. A refrigerator is placed in a room. In this room the transfer of heat out of the room is negligible. If we allow the refrigerator to operate normally, what will happen to the temperature of the room?
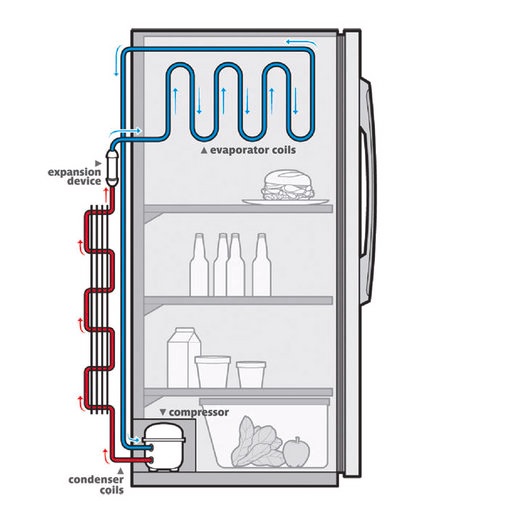
To answer this question we need to take a look at the conservation of energy. First, we know that the refrigerator is designed to remove heat from the air inside the refrigerator and dump it into the room. However, I didn’t say that the refrigerator was perfectly sealed. As a result, heat will re enter the refrigerator from the room. From this you might conclude that the temperature remains the same. However, this is incorrect. The reason why is because electricity used to run the refrigerator is a form of energy. As a result, the room temperature will increase in correspondence to the amount of electricity consumed.