Having an understanding of processes and cycles is an important part of thermodynamics. The reason why is because they are used to describe how a system moves through different states of equilibrium.
Processes
A system that is moving from one equilibrium state to another equilibrium state is under going a process. In addition, as the system moves from these different states it will follow a process path. Finally, to completely define the process, you will need to know what the path is as well as the initial and final states. In addition, you will need to have an understanding of how the system interacts with its surroundings.
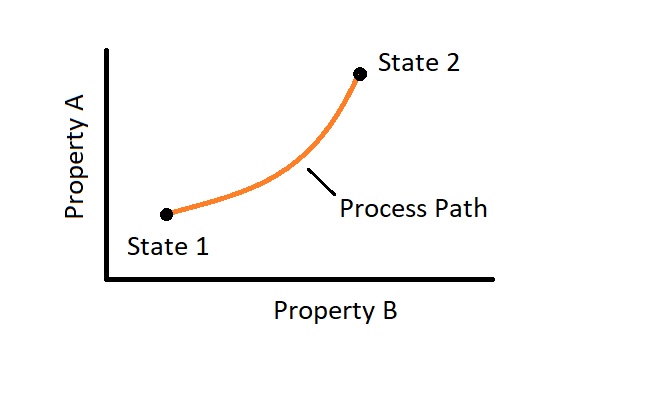
There are different types of processes that can occur. One type is called quasi-static or quasi-equilibrium. In order for this process to occur one part of the system must not change faster than another part of the system. For this to actually occur the process must occur slowly. This process is an ideal process instead of an actual, due to the fact that this process has to occur at such a slow rate. However, many actual process can still be modeled as quasi-static without significant error. The reasons why it is better to model a process as quasi-static, if you can, is because they are easy to analyze, and a process that is quasi-static will deliver the ideal amount work. In turn, a quasi-static process represents a benchmark to base the actual process off of.
Additional processes are isothermal, isobaric, isochoric, and adiabatic. Isothermal means that the temperature will remain constant. On the other hand, isobaric means the pressure remains constant. In addition, isochoric means that the specific volume remains constant. Next, adiabatic occurs when heat doesn’t leave the system during the process. Finally, a cycle occurs when a series of processes occur to bring the system back to its original equilibrium state.
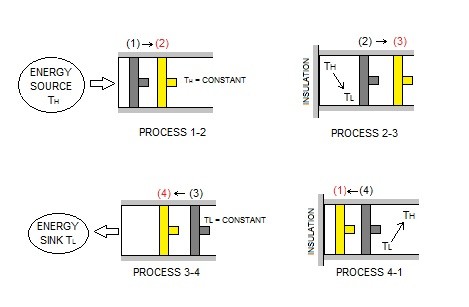
The image above represents a cycle of a piston as it moves through different processes.
Steady-Flow
A steady flow process occurs when a fluid flows through a control volume steadily. In order for this to occur the fluid properties must remain the same at any fixed point. However, the fluid properties can be different at different points within the system.
A steady-flow process is used to model devices that are expected to operate continuously. This includes but not limited to turbines, pumps, boilers, condensers, and heat exchangers.